
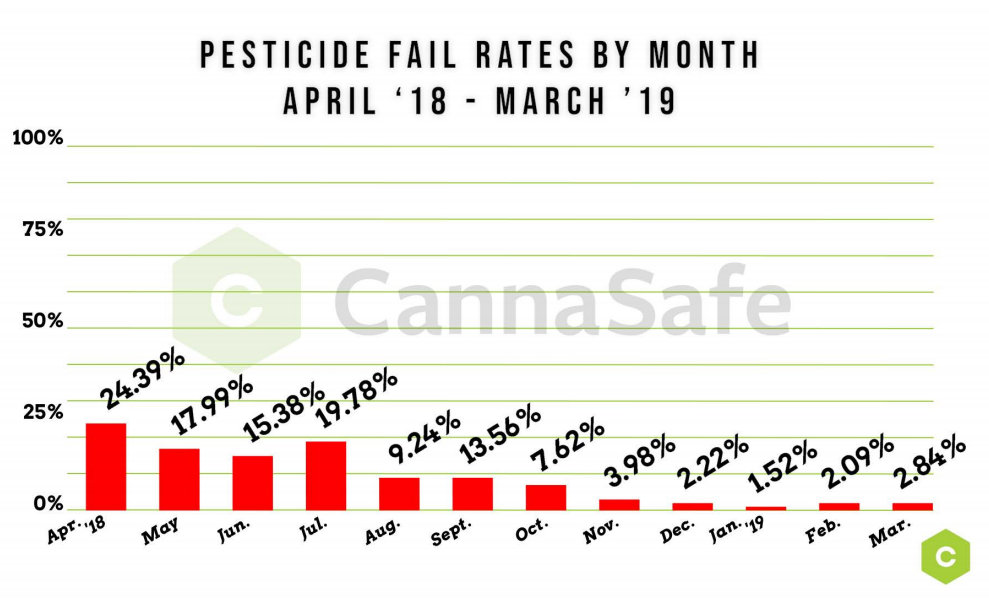
Once viewed merely as a marketing tool by many in the cannabis game, third party lab testing has now become an essential part of the marketplace as new research into the plant is revealing the potential health risks that could come along with cutting corners during the cultivation or manufacturing processes.
Here in California, the state regulatory agencies mandate that all cannabis products be tested not only for potency but also for a wide range of impurities or contaminants including everything from microscopic mold, to unhealthy heavy metals from poor soil or questionable nutrients and pesticides, to residual solvents left over from an extraction process. Any packaged product destined for retail shelves must have attached to it a clean COA (Certificate of Analysis) from a state-licensed lab testing facility.

According to public records shared by the California Bureau of Cannabis Control (BCC), there are 49 active cannabis testing labs licensed by the state. But of those 49, we could only find two that have completed the arduous process of earning accreditation in every facet of the tests they offer – CannaSafe Analytics in Van Nuys, and Anresco Laboratories in LA.

These two labs have the ability to test for all analytes and contaminants as required by the state. They both boast ISO 17025 accreditation that extends across their entire offering of testing services.
The state will eventually require all licensed testing facilities to achieve these high standards, but for now the lab testing aspect of the California cannabis supply chain carries a lot of power without much accountability.
SETTING A NEW STANDARD
With no universal SOP (Standard Operating Procedure) set in regulatory stone, there is currently no mandated baseline by which all testing labs must start from or measure against. There is little or no regulation over where they get their testing equipment or even how they choose to use it. In many cases, testing equipment manufacturers (often with zero experience testing cannabis) are training untested lab staff on how to use the instruments they just bought.
Considering that a failed lab test could result in entire batches of cannabis and related products being destroyed before ever getting to market, or that a faulty lab test could lead to people getting sick, it is more important than ever for everyone from the growers to the consumers to hold cannabis testing labs to the higher standards being set by Anresco and CannaSafe.
We caught up to Zach Eisenberg over at Anresco and asked him for his thoughts on the subject.
Echoing our concerns about SOP’s and variability between results from two different labs testing the same exact batch of buds, Eisenberg told us, “First off, there are no standards for many of the analyses that laboratories are asked to perform. Organizations like ASTM, AOAC, and USP have formed committees to develop consensus standards, but it may be years before they are finalized and available. In lieu of these, laboratories have had to develop proprietary methods and/or defer to publicly available methods that may or may not be fit for use.”
He also mentioned the variation in equipment being used. Just like with any form of technology, there are high end, high quality instruments with incredibly accurate sensitivity settings, and then there’s the cheaper version. Currently, the state isn’t differentiating between the two. It is up to the lab itself to lay out the dough to ensure the necessary accuracy and consistency that only state-of-the-art gear can deliver.
During a recent tour at CannaSafe, for example, we saw one small room alone containing over $2,000,000 worth of liquid chromatograph mass spectrometers and associated gear. But these instruments don’t run themselves, as Eisenberg over at Anresco can attest to.
“There is a human element to what we do. It may take an analyst years to become expert at just a single analysis – not just to learn the method but also to maintain the instrument, troubleshoot issues, recognize the possibility of false positives or negatives, and more,” he explained, adding, “For that reason laboratories are difficult businesses to scale and those that try to grow too quickly or without experienced analysts or procedures in place will have quality issues.”
We have outlined some prime examples of these quality issues in past reporting on the topic.
QUALITY OVER EVERYTHING
At the end of the day, the whole point of third party lab testing is quality control and even the most well-equipped, well-staffed labs in the industry have to deal with variables often overlooked by even seasoned cannabis producers.
 (Photo courtesy of CannaSafe Analytics)
(Photo courtesy of CannaSafe Analytics)
Accredited labs with comprehensive SOP’s go to great lengths to establish homogeneity across the samples they test. But the vast majority of licensed growers and manufacturers are operating on slim or upside down margins these days and, as a result, tend to submit the absolute minimum quantity required to their testing lab. To create a 5 gram sample that is supposed to accurately represent every calyx and pistil in a 10 pound batch is nearly impossible.
Another often misunderstood factor that absolutely impacts homogeneity and consistent test results is the age and condition of the material being tested.
Anyone who has ever grown cannabis has watched the heads of their trichomes gradually merge from crystal clear to an opaque amber hue as the plant ages. Many use this observation to determine the ideal harvest time on specific strains.
 Beard Bros. Pharms Extreme Cream
Beard Bros. Pharms Extreme Cream
Eisenberg sums it up smartly, saying, “The cannabinoid profile of any type of product is going to change over time and the speed of that change will be affected by environmental conditions. So, a potency result for an extract manufactured, tested, and left out in a warehouse for the past three months will likely not be relevant today.”
This is so applicable to today’s cannabis market in California. The 3-phase rollout of testing requirements by the state led to mad rushes of testing, packaging, and selling leading up to the Phase 2 changes in July of 2018, and the Phase 3 changes at the beginning of this year.
Product that didn’t meet those deadlines and was tested at a later date would not only be subject to stricter standards, but would have inevitably changed its cannabinoid profile – even ever so slightly – from when product from the same batch was tested earlier.
So, some onus must be placed at the feet of the growers, manufacturers, and distributors that make up the chain of custody that a cannabis sample travels on its way to a laboratory. How we harvest our plants, how we cure and dry and store them, and how they are processed all impact the final test results that will ultimately define our work.
This is just another reason why working with fully accredited labs is so important.
The ISO 17025 accreditation that labs like Anresco and CannaSafe have earned is a result of them implementing a quality control system meant to improve their ability to consistently produce valid and accurate results.
In other words: Legit SOP’s
You might take all the care in the world to preserve the cannabinoid and terpene profiles in your products, but what if the courier from your local lab leaves them in his trunk on a hot day while he stops for lunch? It may seem petty, but we are learning just how much it matters as testing equipment becomes more and more precise.
The BCC defines a lab quite simply as “A testing laboratory, facility, or entity in the state that offers or performs tests of cannabis goods.”
Attached to that definition is the disclaimer: Testing laboratories must obtain and maintain ISO/IEC 17025 accreditation. Testing laboratories may be issued a provisional license allowing them to operate while they obtain ISO/IEC 17025 accreditation, provided they meet all other licensure requirements.
Currently, every active lab license listed by the BCC is labeled as “temporary”, with all of those temps due to expire throughout 2019. Labs like Anresco and CannaSafe will, presumably, transition seamlessly into an annual license, but other labs that have not completed the accreditation process could be left in limbo, along with any loyal customers they may have.
“It is certainly a positive development that ISO 17025 accreditation will be required of all cannabis laboratories in California,” says Eisenberg at Anresco, “It will force the labs to formalize their methods, participate in check sample programs, develop SOP’s, undergo routine quality audits, and more.”
He adds, “Accreditation will not completely eliminate lab variability or bad actors, but it will help. The result should be more meaningful test results and subsequently safer products, which will benefit both consumers and the industry as a whole.”
Now those are the results we’ve been waiting for.
Keep updated on all the latest news and updates in the Cannabis industry here at Beard Bros Pharms by signing for our Friday Sesh Newsletter here. Always Dank and Never Spam!
















