
Here in California, we’ve been told time and again that the main benefit that the regulated cannabis market has over its street market competition is the fact that all regulated/taxed cannabis is subject to rigorous third-party laboratory testing to ensure that it is safe to consume and is accurately labeled.
As a licensed distributor and manufacturer ourselves, and in our role operating licensed cultivation facilities, we at Beard Bros. Pharms rely heavily on third-party lab testing to validate the products that we make so that the state will allow them onto retail shelves. Those retail cannabis dispensaries also rely on the validity of third-party lab testing as they are the ‘face’ that many consumers are going to associate with the brands bought there.
Testing labs are tasked with analyzing samples of buds, edibles, extracts, and everything else that you find on the shelves of a well-lit weed shop to determine if those batches contain any banned contaminants like residual solvents, pesticides, or molds, to name just a few.
The California Bureau of Cannabis Control (BCC) currently lists 36 “Active” testing labs in its license search tool. As with any link in the cannabis supply chain, obtaining local authorization and a state license to open a testing lab is not cheap or easy – up to $112,000/year! Still, we feel that state regulators are taking it a little too easy on some labs by allowing them to “phase in” their ISO/IEC 17025 accreditation requirements.
This means that some labs are still running tests and providing results for aspects of the testing process that they have not been independently audited/accredited for yet. These and other loopholes need to be addressed in order to ensure products are safe to consume and operators are competing on a level playing field.
PHASING IN…DEFINITELY

The BCC requires that labs maintain an ISO/IEC 17025 scope of accreditation for all of the compliance tests that they offer. ISO 17025 is not only an industry standard, it is an international standard used to evaluate the technical competence and quality control measures in testing and calibration laboratories. Third-party auditors perform these evaluations on a regular basis to ensure that labs uphold these high standards in order to maintain their state-mandated accreditation.
Though it varies a bit by product type, the BCC clearly defines exactly what tests must be performed in order for a batch of cannabis product to be deemed compliant. The problem is, over two years into California’s cannabis testing regulations, the state is still allowing labs to “phase in” those accreditation requirements. As it stands now, if you go to apply for an interim testing lab license here in Cali, you just need to attest that you “intend to seek ISO/IEC accreditation for all testing methods”.
If you are granted that interim license, it is valid for 12 months. At that point, you can renew that same interim license for an additional 12 months, regardless of where your lab stands with ISO/IEC accreditation. Only then, after a full two years (if not longer), will the state ask to see that the lab is pursuing third-party accreditation. For two years or more a lab can be performing compliance tests outside the scope of their ISO accreditation status.
This “phase in” loophole appears to have some labs more motivated than others to get it done. This increases the odds of product landing on dispensary shelves that may or may not have accurate labeling or be truly compliant, or even safe, to consume.
The reason why the BCC phased in these requirements is that in 2018, there were not enough accredited laboratories to perform all the compliance testing required, and they wanted to give existing labs time to come into compliance. Again though, we are two years out and those capacity concerns are no longer valid. Our research indicates that fully accredited laboratories can more than handle the 1,300 or so compliance submissions made to the BCC each week.
We decided to go straight to the source and ask the people operating California’s top licensed testing labs for their take on ISO/IEC accreditation and if/why it matters. Aaron Riley, the founder and CEO of Los Angeles-based Cannasafe lent us his perspective in a recent interview, saying, “Third party accreditation is one of the most important ways make sure you’re doing things right. We became accredited for heavy metals almost a year before regulations hit because we wanted to be ahead of any hiccups that most labs are facing now.”
As a proud member of the Patients Focused Certification program overseen by Americans for Safe Access, Cannasafe was among the first labs in the state to achieve full ISO/IEC 17025 accreditation and they display their current certificates right on their website.
Not all labs here in California make the scope of their ISO/IEC accreditation available to the public. What we were able to find initially raised quite a few red flags, but after following up with several high-volume labs, we were glad to learn that most have made significant advancements in their accreditation status – some while we were in the process of writing this article, in fact.
Still though, this “phase in” loophole exists and is being exploited in what we call…
THE PESTICIDE PROBLEM
For most contaminants, the BCC sets an “action limit”.
Test above the limit and the batch fails.
Test below the limit and it passes.
To the Bureau’s credit, California’s action limits are the lowest in the country when it comes to cannabis testing, and labs here must be geared with state-of-the-art Gas Chromatography and Liquid Chromatography instruments in order to accurately analyze samples at that level.
However, for Phase I pesticides the BCC set the pass/fail criteria at the lab’s limit of detection (LOD) or the lowest value at which the lab can identify the presence of an analyte. Here is the relevant text:
§ 5719. Residual Pesticides Testing. (a) The laboratory shall analyze at minimum 0.5 grams of the representative sample of cannabis goods to determine whether residual pesticides are present. (b) The laboratory shall report whether any Category I Residual Pesticides are detected above the limit of detection (LOD) and shall report the result of the Category II Residual Pesticides testing in unit micrograms per gram (µg/g) on the COA. The laboratory shall indicate “pass” or “fail” on the COA.
(1) The presence of any residual pesticide listed in the following tables in Category I are not detected, and
(2) The presence of any residual pesticide listed in the following tables in Category II does not exceed the indicated action levels.
The problem is LOD values vary depending on the product being tested, the method employed, and the instrument used. This provides labs a lot of leeway as to what they consider a pass, especially if the lab is not accredited and the method is not audited. That means that pass/fail criteria vary depending on which lab you use.
Unfortunately, a substantial number of growers and manufacturers will inevitably swarm to labs with the highest LODs in order to reduce their chances of a costly Failed lab test.

This loophole could be choked, or maybe closed completely, with state-mandated action limits on all Category I Pesticide analytes.
For more on this facet of the problem, we reached out to Zach Eisenberg at Anresco Laboratories.
“The BCC regulations of Category I Pesticides as written are problematic. The text states that a batch only passes if all ‘residual pesticide[s] listed below in Category I are not detected’. However, each laboratory is permitted to set their own limits of detection (LODs), so what passes at one lab might fail at another,” Eisenberg told us, confirming our suspicions.
Eisenberg continued, “This system does not make sense because labs with better methods or instrumentation that achieve lower LODs are at a competitive disadvantage. Customers are incentivized instead to send samples to labs with higher LODs which are less likely to fail their batches.”
Around the end of 2019, we gathered a slew of testing data from industry peers and we recently took a hard look at the LODs on Category 1 Pesticides from six different California state-sanctioned testing labs.
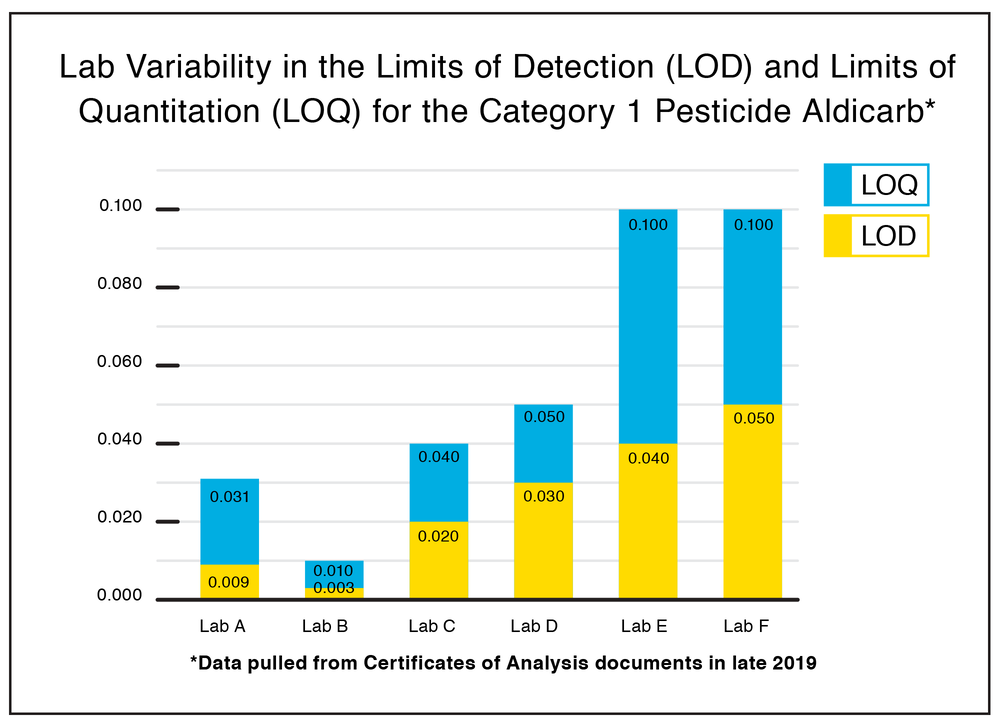
Aldicarb is one of 21 Category I pesticides. The compound is a highly toxic insecticide considered to be an “extremely hazardous substance” here in the U.S., subject to strict reporting and record-keeping practices, and not approved for use on foods.
Though Alidcarb is applied to cannabis crops to limit aphids, leaf-miners, thrips, and spider mites, it can also be deadly to mammals, from rodents up to humans. In South Africa, for example, burglars use Aldicarb to poison watchdogs. It targets the respiratory system, paralyzing it – hardly the sort of thing you want to be ingesting or inhaling. Additionally, the high solubility and runoff of Aldicarb into water tables, creeks, and streams has been attributed to the destruction of entire ecosystems.
The chart above shows how much wiggle room there is between just six labs here in California when it comes to the allowable amount of this dangerous and unnecessary pesticide.
Some labs that had comparatively low LODs on some analytes had inexplicably raised those numbers for the most commonly discovered (and still banned) pesticides. Many California labs, for example, allow a LOD of .05 on Chlorfenapyr.
Chlorfenapyr has been banned for use on food crops because it is highly toxic to humans and animals when consumed orally. 31 people died in Pakistan in 2016 when a food supply was spiked with this pesticide. So, let’s smoke more of it? That makes no sense. Nor does it make sense that every lab can have a different number.
Clearly, some labs are trying to woo clients in with those higher limits and it is working. Lab shopping is not just for THC anymore, fam!
The good news is, a growing majority of the 36 Active cannabis testing labs in California have achieved full ISO/IEC 17025 accreditation, and most are being fully transparent with that information. This is why we are confident that those who have completed their full accreditation can handle the current market demand for compliance COAs.
That still doesn’t go quite far enough, though, in our opinion. Even once every licensed testing lab is fully accredited, there still needs to be uniform action limits, ensuring that some labs aren’t passing higher levels of Category 1 Pesticides than others.
Aaron Riley at Cannasafe went hard on this topic in our discussion, telling us, “Our goal is consumer safety. If you lose the trust of the consumer by threatening their safety, then it’s game over. We’ve had clients that went lab-shopping and left us; we would get a call a month later saying their lab was shut down by the BCC for not following regulations or falsifying results and they need us to pick up samples. Most of the time it’s not even worth it.”
The BCC cannot claim ignorance here as they have already addressed the same exact issue when it comes to residual solvents. Zach Eisenberg at Anresco explains, “The BCC recognized this problem for Cat I residual solvents and set action levels for these so all labs could operate on a level playing field. Inexplicably they did not do the same for Cat I pesticides.”
Here’s the exact text in the final regulations that he’s referencing (emphasis ours):
Subsection (d) is now subsection (c) and has been amended by specifying that a sample shall be deemed to have passed the residual solvents and processing chemicals testing if the presence of any residual solvent or processing chemical listed in the tables in Category I and Category II does not exceed the indicated action levels. The proposed language would have required testing laboratories to establish a limit of quantification (LOQ) of 1.0 μg/g or lower. The Bureau received numerous comments that the proposed language is arbitrary and that it increases the variability in testing results from one laboratory to the next. Numerous commenters specifically requested that the Bureau establish specific action levels for Category I solvents, rather than allowing laboratories to establish a LOQ on their own. Thus, the Bureau determined that specific action levels are necessary to ensure standardization across the licensed laboratories.“
Unwilling to allow labs to establish their own potentially unsafe LOD/LOQ on solvents, the state instead sets those limits itself, establishing a baseline by which all labs must abide by. The same must be done for pesticides. This would create a true Pass/Fail scenario consistent statewide across all labs and would encourage cannabis testing labs to focus on consumer safety instead of reducing the quality of their results.
Imagine that!
TOTAL RECALL
As state regulators drafted and re-drafted the rules around lab testing on their path to the Phase 3 testing standards that we have now, some industry insiders feared that stringent new guidelines could initiate an “extinction event” in the state’s young cannabis industry if growers, extractors, and manufacturers couldn’t dial in their products.
The fear was born from the costly repercussion that comes with a failed lab test here in Cali – the potential for mandatory destruction of the entire associated batch of product. A few grams of failed flower could mean the loss of a 50lb batch of buds.
Fortunately, that particular extinction event didn’t happen (yet), but there have been some brand-crippling and career-ending recalls in the past couple of years under Prop 64, and there has been a fair share of fuckery by testing labs at the root of them.
In 2018, 29 different brands were informed by the BCC that they would face a mandatory recall of thousands of products already sold to retailers after Sacramento-based Sequoia Analytical was caught falsifying pesticide testing results on potentially hundreds of batches of flower, pre-rolls, edibles and more.
At the time, it was reported that “tens of millions” of dollars’ worth of products would be recalled. Of course, the companies being forced to take the product back could draft a Corrective Action Plan and submit that to the state asking for permission to remediate the products. While they wait, they need to store those products in a separate “Quarantined” area. Then, if their Corrective Action Plan was approved, they could have it re-tested elsewhere and then re-sold assuming it passed, but all of those costs of repackaging and retesting would fall onto the shoulders of the licensees who got screwed by the offending lab.
The entire point of state-mandated lab testing is to shine a bright light on quality control and provide transparency for all stops of the supply chain, including and most importantly for the end-user. Instead, the state appears to be giving some labs a large shadow to operate in by ‘phasing in’ the mandate for full ISO/IEC 17025 accreditation for all services that they advertise and provide.
Furthermore, the BCC needs to do a much better job of informing the entire supply chain anytime a lab has had its licensure detrimentally impacted by the state – even if it is temporary. There are too many stories of what is likely perfectly compliant product being held up due to improprieties occurring in the lab, and we usually learn about them after the fact, through word of mouth rather than any official announcement.
Recalls are a rare and extreme example, though. More common is another topic we have covered before…
THC TESTING IS BULLSHIT AND YOU’RE GETTING SCREWED
We have been in the weed game for a while, but the regulated market is new to all of us. Selling weed in bulk used to be a pretty straight-forward process – years of street precedent, along with your reputation and the bag appeal of the buds, typically set the price. Lab testing back then was rare, voluntary, and most often seen as more of a marketing tool than an educational one. Little did we know just how much influence it would come to have on hype and pricing.
These days, way too many purchasing managers in the regulated market are basing their buying on nothing more than the total THC found in the product. The higher the THC on the lab test, the higher the wholesale (and ultimately retail) price will be at many shops.
This is not only incredibly lazy, and incredibly ignorant of the importance of a full and balanced spectrum of cannabinoids (and terpenes, and flavonoids, and…), but it sets a dangerous precedent for labs to hit ever-rising numbers for pushy clients trying to get top dollar for their goods.
Lab test failure rates have steadily declined statewide over the past year or so, but potency totals have risen just as steadily. It makes sense that growers, extractors, and manufacturers would quickly dial in their own SOPs to create compliant products, but how are they suddenly boosting THC production to never-before-seen levels?
It’s bullshit, and chances are you’re getting screwed. Labs in we have covered before…, Nevada, and Oregon have already been caught inflating numbers and labs in California are probably doing it too.
THE BOTTOM LINE ON CANNABIS LAB TESTING
The point of the article is that distributors and other licensees on the cannabis supply chain need to be more discerning when choosing a lab to work with. Easier is not always better, and it is rarely safer.
We want to believe that all licensed labs are taking the necessary steps to achieve full accreditation and set safe standards and we will be happy to update this story with any new information shared with us.
Again, the entire point of third-party lab testing is quality control. Any lab that can point to a full ISO/IEC 17025 accreditation for all services that they offer demonstrates that their own level of quality control is on point. It demonstrates that even though the state doesn’t require them to employ sensible standard operating procedures, they take it upon themselves to do so anyway.

That attention to detail will absolutely carry over to the accuracy of the test results of your product and if you do things right, that’s all you should ever ask a lab to provide.
















