Aspergillus Testing in the United States – An Overview, Insights, and Perspectives is Written by Kyle Boyar, Field Application Scientist – Cannabis North America
Bio-Rad Laboratories, Food Science Division
In a testing sector that is often heavily focused on chemistry and THC values, microbiological testing for cannabis and cannabis products is often overlooked. However, it is one of the most important kinds of analysis in the sense that exposure to certain contaminants can be fatal for certain populations. One of the most heavily criticized analyses in cannabis microbiology is Aspergillus testing which has been a source of controversy in the cannabis industry in recent years despite its clinical relevance 1-11. Many jurisdictions in the US currently test for four species of pathogenic Aspergillus spp. as a requirement for inhaled cannabis products across 24 US states.
This article will describe how this kind of testing is performed, the differences between commonly used methods, and various aspects that could lead to discrepancies in test results. This article will also provide perspectives on best practices for Aspergillus testing and how to ensure products are not failing needlessly while still promoting consumer safety.
How is Aspergillus Testing Performed?

In most jurisdictions with Aspergillus testing, four different species, A. flavus, fumigatus, niger, and terreus are examined using a method called quantitative polymerase chain reaction, or qPCR. The process starts by obtaining a representative sample of the test batch. To keep this simple, I will use California’s regulations as the example which utilizes what many would consider good practices in terms of statistical relevance.
Keep in mind that sampling protocols and requirements vary widely across the US, and some do a better job of this than others. In California, a representative sample is obtained by taking 0.35% of a batch which is taken from different sections known as increments12. You can think of increments as creating a grid across the batch and taking from each different square within that grid. The number of increments required varies by the batch size as seen in the figure below.
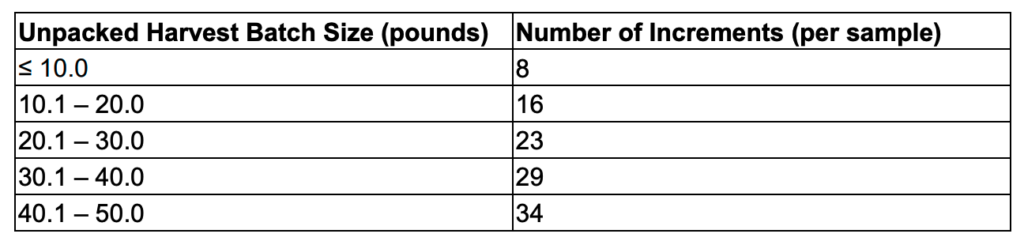
Using a fifty-pound batch as an example, this would require that the sampler obtain 79.37 grams taken from 34 different increments. The sample is then homogenized accordingly to achieve an accurate representation of that batch.
From this homogenized test batch, a one-gram sample is then aseptically taken from and placed in a filtered bag which is then diluted with a sterile media which contains everything needed to encourage the growth of the target microorganism. Oftentimes, this is done with something like buffered peptone water (BPW) or tryptic soy broth (TSB) and these are usually supplemented with an antibiotic to knock down background flora that could outcompete the target organism. The sample is then placed in an incubator at 37 C in a process known as enrichment. You can think of enrichment as the difference between trying to find a needle in a haystack versus finding thousands of needles in that same haystack. Enrichment times vary depending on the testing platform used however the United States Pharmacopeia (USP) recommends a 48-hour enrichment time 13.
Once the enrichment has been completed, the enrichment bag is removed from the incubator and a 1mL subsample from the enrichment bag is used for subsequent DNA extraction. Depending on the method, the entirety of the 1mL subsample is analyzed but some methods reduce this down to as low as 1/5th of the volumes. For some methods like the end-point PCR coupled with microarray, the actual amount analyzed is significantly lower because this technique operates differently. For sake of simplicity, this article will focus on the most widely accepted methodology, qPCR. There are a variety of extraction methods, but most involve some form of chemical treatment or heat that causes the cells in a sample to rupture which is known as a lysis step. Once the cells have been ruptured, the DNA is now exposed and can be utilized in the downstream qPCR reaction.
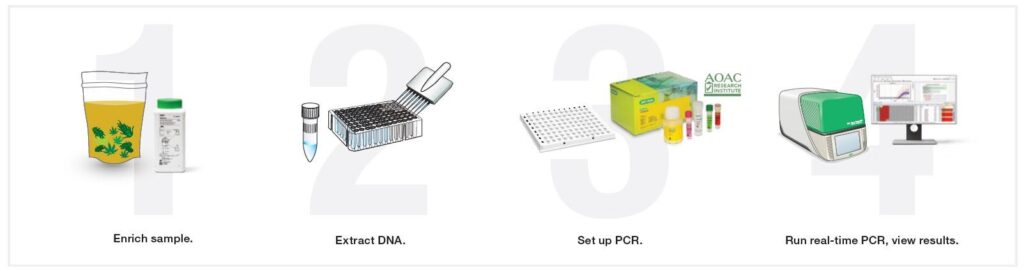
Once the DNA has been extracted, a small amount of this is added to each well combined with something known as master mix into which contains all the necessary components for PCR to occur (primers/probes, dNTPs, Mg2+, DNA polymerase, etc). These reactions are placed into a 96-well plate, sealed with optical film, and inserted into the instrument for cycling. Cycling involves the heating and cooling of the sample for 40-50 cycles. Each time this occurs the target DNA sequence, if present, increases exponentially. This exponential increase in target DNA is coupled to a release in fluorescence by the probe and this fluorescence is what is read by the instrument.
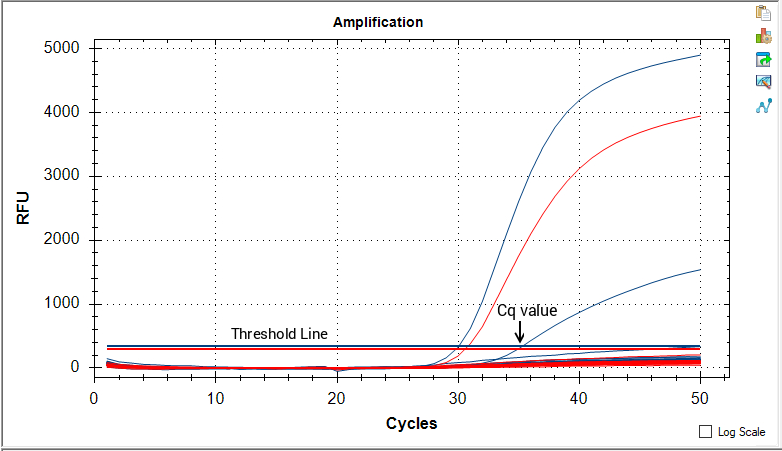
A threshold line is then set manually by the user or by the instrument software and the point at which fluorescence crosses the threshold line is known as a cycle of quantitation or Cq value. Cq values are inversely correlated with the amount of target DNA present, meaning a lower Cq value has lots of the target DNA, while a higher value means it has less. If the fluorescence crosses threshold, the sample is considered not passing and must either be destroyed or remediated.
Differences in Aspergillus Testing Methods
Enrichment Media – Most platforms utilize buffered peptone water (BPW) or tryptic soy broth (TSB) as their enrichment media. As mentioned above, this enrichment media is often supplemented with an antibiotic such as chloramphenicol or chlortetracycline to knock down any non-target organisms (i.e. bacterial background flora). This ensures that the other background flora do not interfere with the detection of the fungal target. Not all methods utilize an antibiotic which could lead to out competition by other organisms within a sample
Enrichment Times – Most platforms utilize 24–48-hour enrichment times however some platforms do not enrich at all and use a similar but different technology known as end-point PCR coupled with a DNA microarray. Due to a lack of enrichment, this kind of methodology is sometimes not accepted in certain states.
Subsampling – To achieve sample representation, typically a 1mL subsample is taken from a 1:10 dilution (1 gram sample, 9 mL of enrichment media). However, during DNA extraction there are often several manipulations that can occur that lead to a range from 20%-100% of the 1mL subsample being analyzed.
Live/Dead Analysis & Free DNA Removal – To ensure that only DNA associated with live cells is being amplified most vendors have implemented a step to remove DNA from dead cells. This can either be in the form of enzymatic solutions in which a DNA degrading enzyme is applied prior to lysis or in the form of DNA intercalating dyes which bind to the DNA in dead cells rendering them non-amplifiable.
Cell Rupturing (Lysis) – Nearly all methods use some chemical means to rupture Aspergillus cells. Sometimes this is coupled with heat, shaking, or mechanical mechanisms such as silica beads. Some methods utilize a crude DNA extract without any additional clean-up while others have additional purification steps such as the use of DNA binding magnetic beads.
Primer/Probe Sets – qPCR utilizes primer pairs designed to anneal (bind to) upstream and downstream of the target sequence. In the context of Aspergillus testing, designing these primers to achieve specificity is notoriously difficult. It is important to note that to date, there are no platforms on the market that can achieve 100% specificity for the four target species of Aspergillus. Cross-reactivity with closely related species is seen in all platforms.
Speciation – Some vendors have 5-color assays which allow for differentiation of the various species upfront while others do this separately with individual primer sets in the event of a positive hit on the initial screen. Each of these approaches have their own advantages and disadvantages in terms of cost and time.
Cycling Times: Some vendors have shortened their qPCR cycling times to gain a market edge in terms of turnaround time. Simultaneously, this shorter cycling time can also lead to a lower likelihood of DNA amplification to occur to a level that would cross the threshold.
General Issues with Aspergillus Testing
Beyond the differences in testing platforms listed above, Aspergillus tends to form clumps not only within a batch but also within the enrichment bag. If a sample is not well mixed prior to subsampling, this clumping can cause significant challenges when trying to achieve concordance even within the same enrichment. Furthermore, utilizing a one-gram sample is hardly representative of an entire batch. In other industries such as food testing, typically a minimum of 25 grams is utilized. However, this isn’t entirely an apples-to-apples comparison. Inhaling 1 CFU/g of Aspergillus in most healthy people is unlikely to cause disease, while ingesting 1 CFU/g of Salmonella in most healthy people could lead to severe illness.
Aspergillus Detection Rates Across the United States
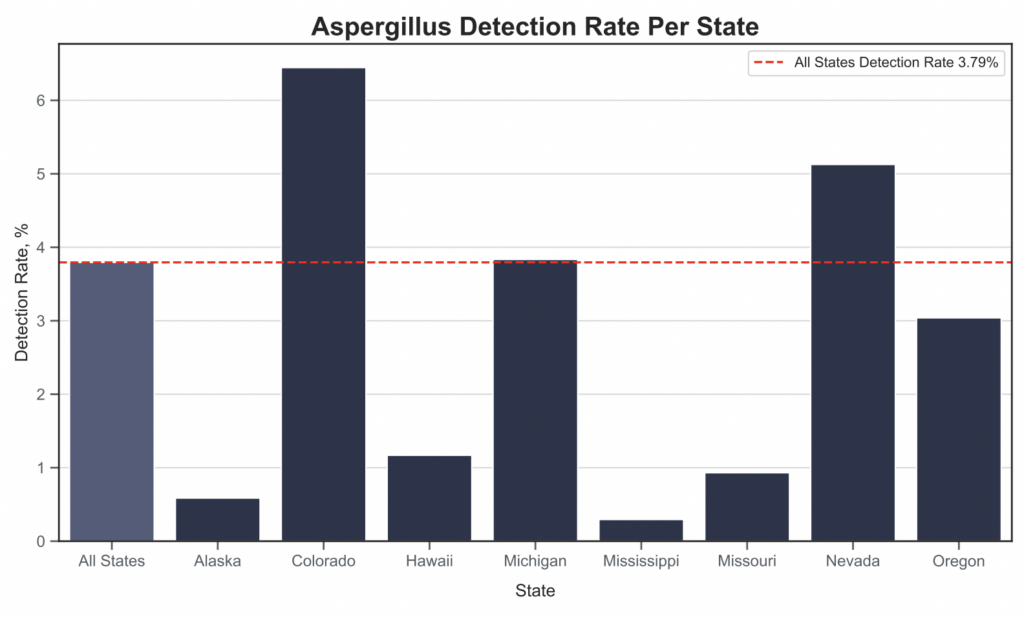
Now that we’ve gone over the process and the differences between testing platforms, how does the data look across different parts of the US? The figure below yields some interesting insights, some of which are entirely counterintuitive (i.e. little detections in warm humid environments in which Aspergillus would typically thrive). However, before making any conclusions, it is important to take all the factors above into consideration.
Suggestions for Improving Aspergillus Testing
Below are a couple of opinions on what could be done better in the context of Aspergillus testing. By implementing these changes, the industry could save millions of dollars by avoiding the needless failure of tens of thousands of products while simultaneously optimizing consumer safety.
Problem #1: Cultural confirmation is not a requirement and is hardly ever performed
At present, many state regulations consider the presence of any amount of target Aspergillus DNA present to be considered a failing result. This is entirely the wrong approach as even a few dead cells of Aspergillus pose very little if any risk to the consumer and can result in a failing product. In nearly all other established industries such as food and environmental, a positive on PCR is considered what we call a “presumptive positive”, however a sample is not a true positive until it has been culturally confirmed via plating methods (Saboraud Dextrose Agar, Potato Dextrose Agar, etc).
By culturally confirming, this demonstrates that there are actual viable organisms within the sample. Dead organisms, aside from their potential byproducts, are not cause for concern. Since plating Aspergillus spp. can take up to five days and many cannabis producers want their results yesterday, they oftentimes will push labs to release results as soon as possible and disregard best practices which is in this case means enriching for the full 48 hours and opting for cultural confirmation to demonstrate viability, and hence demonstrating any kind of potential risk.
However, this is not always the fault of labs as some jurisdictions simply will not allow for cultural confirmation. This cultural confirmation step is essential especially considering the amount of remediation techniques that are currently being employed in the cannabis industry (i.e. irradiation, ozone, UV, etc) 14. However, as mentioned earlier, one caveat is that these remediation techniques come with their own risks as many harmful byproducts such as endotoxins, allergens, and mycotoxins can be left behind on the remediated product therefore these are not a failsafe in the context of consumer safety.
Problem #2: We’ve applied the same regulations for recreational and medical users
We have done medical cannabis patients a tremendous disservice by having the same testing regulations for both recreational and medical patients. These are entirely different populations with entirely different needs. In general, those who are immunocompromised should not be smoking or vaporizing anything, but cannabis for medical use should have as low of a bioburden as possible when being used by medical patients as they are the most prone to opportunistic infections due to compromised immune function.
Conversely, recreational users typically have healthy and well-functioning immune systems and often have no issues inhaling hundreds of Aspergillus spores daily, however, these not always of the pathogenic kind. This is evidenced by several publications that show that aspergillosis is most associated with those compromised immune function but not out of the question for the immunocompetent 1-11, 15-16. By setting the bar so high for recreational markets, we’ve likely endangered people further as this encourages the use of fungicides to achieve a passing result. This also can lead to the misuse of remediation techniques which is often used to “recover” heavily contaminated product
Conclusion
A balance needs to be struck and what that looks like at present is not entirely clear. As highlighted in the example comparing cannabis testing to food testing, it is imperative that we reevaluate how we approach Aspergillus testing to avoid unnecessary economic hardship. One option would be to set a threshold of allowable Aspergillus for recreational users while keeping the current non-detect requirement specific to medical cannabis. It is my hope that this article sparks more of these discussions so that regulatory bodies can better determine what constitutes reasonable policy for this kind of testing and help alleviate those who have suffered losses from existing policies.
Acknowledgments
Bio-Rad Laboratories, Food Science Division
Yasha Kahn, MCR Labs
References
- Remington TL, Fuller J, Chiu I. 2015. Chronic necrotizing pulmonary aspergillosis in a patient with diabetes and marijuana use. CMAJ 187:1305–1308.
- Hamadeh R, Ardehali A, Locksley RM, York MK. 1988. Fatal aspergillosis associated with smoking contaminated marijuana, in a marrow transplant recipient.Chest 94:432–433.
- Cescon DW, Page AV, Richardson S, Moore MJ, Boerner S. 2008. Invasive pulmonary aspergillosis associated with marijuana use in a man with colorectal cancer. Journal of Clinical Oncology 26:2214–2215.
- Chusid MJ, Gelfand JA, Nutter C, Fauci AS. 1975. Pulmonary aspergillosis, inhalation of contaminated marijuana smoke, chronic granulomatous disease. Annals of Internal Medicine 82:682–683.
- Llamas R, Hart DR, Schneider NS. 1978. Allergic bronchopulmonary aspergillosis associated with smoking moldy marihuana. Chest 73:871–872.
- Verweij, P. E. (2000). Fungal Contamination of Tobacco and Marijuana. JAMA, 284(22), 2875. doi:10.1001/jama.284.22.2869
- Marks WH, Florence L, Lieberman J, Chapman P, Howard D, Roberts P, Perkinson D. 1996. Successfully treated invasive pulmonary aspergillosis associated with smoking marijuana in a renal transplant recipient. Transplantation 61:1771–1774.
- Salam AP, Pozniak AL. Disseminated aspergillosis in an HIV-positive cannabis user taking steroid treatment. The Lancet Infectious Diseases. 2017;17(8):882.
- Kagen S. 1981. Aspergillus: an inhalable contaminant of marihuana. The New England Journal of Medicine 304:483–484
- Gargani Y, Bishop P, Denning DW. 2011. Too many mouldy joints—marijuana and chronic pulmonary aspergillosis. Mediterranean Journal of Hematology and Infectious Diseases 3:e2011005.
- Bal A, Agarwal AN, Das A, Suri V, Varma S. 2010. Chronic necrotising pulmonary Aspergillosis in a marijuana addict: a new cause of amyloidosis. Pathology 42:197–200.
- California D of CCS of. DCC regulations. Department of Cannabis Control. https://cannabis.ca.gov/cannabis-laws/dcc-regulations/ Accessed November 9, 2024
- Sarma ND, Waye A, ElSohly MA, et al. Cannabis inflorescence for medical purposes: usp considerations for quality attributes. J Nat Prod. 2020;83(4):1334-1351.
- Frink S, Marjanovic O, Tran P, et al. Use of X-ray irradiation for inactivation of Aspergillus in cannabis flower. PLoS One. 2022;17(11):e0277649.
- Babu T, Griswold MK, Urban MA, Babu KM. 2017. Aspergillosis presenting as multiple pulmonary nodules in an immunocompetent Cannabis user. Journal of Toxicology and Pharmacology 1:004.
- Zaga et al. Invasive pulmonary aspergillosis in an immunocompetent, heavy smoker of marijuana with emphysema and chronic obstructive pulmonary disease.Canadian Journal of Respiratory, Critical Care, and Sleep Medicine, 2020, 400-403























